(Click here to read our disclaimer)
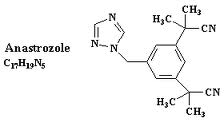
Anastrozole is commonly produced under the name Arimidex. This chemical is not naturally occurring and is currently not available outside of laboratory settings. Scientists are currently investigating this chemical’s impact on estrogen receptors or conversion mechanisms– both in an artificial and natural state.
Anastrozole was designed to act as an aromatase-inhibiting chemical that may have applications toward metastasis. Potential use in combatting the effects of hyperplasia is currently being studied, as is the reactions of anastrozole when interacting with the synthesis of estrogen.
The patent on this chemical expired in 2010 which has opened the door for additional testing and research on these effects.
Chemical Mechanisms
Anastrozole is prepared in laboratory environments by subjecting mesitylene to a radical brominisation process.
- Anastrozole is prepared by substituting two benzylic for cyanide, treating the chemical with potassium cyanide using a phase transfer condition.
- The methylation of this chemical with sodium hydridie and methyl iodide creates an acidic compound that includes several methyl groups and hydrogen atoms.
- Treating bromine along with benzyl peroxide will create a corresponding benzyl bromide reaction that creates the base for the aromatase inhibitor.
Additional compounds can be created using a similar process. This allows manufacturers to create variations on the compound to increase or decrease the strength of the chemical as necessary. Varying compositions of anastrozole can be used in laboratory settings to act as carriers or diluents based on the nature of the study.
Study
Studies are ongoing to determine the usefulness of using anastrozole for medical application in the future.
Ongoing studies are in progress to assist in determining the inhibiting properties of anastrozole as well as the process of compounding these chemicals to create a variation of strengths of this chemical.
Click here to view our entire PDF research library
Click here to view/download the PDF version of this article